Mast cells (MCs) are multifunctional, tissue-dwelling cells .
In the bone marrow, MC-committed progenitors begin their differentiation under the influence of growth factors; then, they migrate in the bloodstream and reach various body sites
In these tissues, MC-committed progenitors mature under the influence of stem-cell factor (SCF) in humans and additionally, interleukin-3 (IL-3) in rodents.
Interestingly, mature MCs are distributed throughout connective and mucosal tissues, where they interface with the external environment
When MCs are activated, they extrude granule-associated substances, such as histamine, immediately and within minutes, generate lipid-derived mediators . MC activation is followed also, within hours, by the de novo synthesis of numerous cytokines and chemokines . Inflammatory mediators produced by MCs are best known perhaps for their initiation and maintenance of anaphylaxis, as well as their role in other pathological conditions, including rheumatoid arthritis, interstitial cystitis, scleroderma and Crohn’s disease
Interestingly, despite the detrimental effects of their mediators, MCs have been described in the lowest order of animals (e.g. insects and fishes). Their persistence through evolution suggests a beneficial and important role for these cells in the body.
The preferential location of MCs at the portals of entry of many infectious agents ensures the early contact of MCs with invading pathogens MCs seem to be particularly important for mounting efficient innate immune responses against bacteria, through their release of proinflammatory and chemotactic mediators.
Table 2. Major characteristics of the two mouse mast-cell subtypes

Abbreviations: ABS, alcyan blue safranin; CS, chondroitin sulfate; CTMC, connective-tissue mast cell; MMC, mucosal mast cell; MMCP, mouse mast-cell protease.
b Symbols: +++, the stimulator causes maximal release of mediators by MCs; ++, the stimulator causes sub-maximal release of mediators by MCs; +, the stimulator causes limited but significant release of mediators; +/-, the stimulator leads to very limited release of mediators; -, the condition is unable to stimulate MCs.
a Abbreviations: MCT, mast cell containing only tryptase; MCTC, mast cell containing tryptase and chymase.
b Data are based on our unpublished results and Refs [1, 7 and 20].
c Symbols: +++, the stimulator causes maximal release of mediators by MCs; ++, the stimulator causes sub-maximal release of mediators by MCs; +, the stimulator causes limited but significant
MC recognition and killing of opsonized bacteria
During invasion of a healthy host, the pathogen is opsonized frequently by natural antibodies and/or complement components. MCs can recognize and attach to a wide variety of opsonized bacteria. In rodent MCs
Salmonella typhimurium coated with the iC3b fragment of complement is recognized through complement receptor 3 (CR3) on the MC membrane.
MCs express several IgG receptors that are involved in the binding of IgG-coated bacteria to the MC membrane. Following their binding to MCs, opsonized bacteria are phagocytosed.
Various strains of Escherichia coli, Enterobacter cloacae and Klebsiella pneumoniae bind avidly to mouse BMMCs in opsonin-dependent conditions
Evidence for an oxidative bactericidal system in MCs comes from the substantial oxidative burst generated by MCs upon exposure to Gram-negative bacteria in opsonizing conditions
In contrast to the large amount of data generated using rodent MCs, limited data exist describing the interactions of human MCs with bacteria. Recently, we have shown that human cord-blood-derived MCs (CBMCs) and MCs from the human leukemic cell line HMC-1 bind and internalize several types of opsonized Gram-negative and Gram-positive bacteria and This results in a significant loss of bacterial viability .
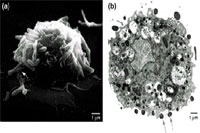
Fig. 1. (a) Scanning electron micrographic and (b) transmission electron micrographic examination of human cord-blood-derived mast cells (CBMCs), after a three-hour exposure to a noninvasive strain of Escherichia coli in opsonin-dependent conditions. (a) The cell surface of the CBMC is covered with several adherent bacteria. Notice that bacteria appear, in some cases, to be gripped by filapod-like structures (white arrow). (b) Several E. coli are associated with the membrane of the CBMC (gray arrows). In the same CBMC, some of the intracellular bacteria appear to be degraded (black arrows).
לחץ על התמונה להגדלה
MC mediator response to microorganisms
An important result of the interactions of opsonized bacteria with MCs is the secretion of various mediators by MCs.
MC products elicited by the binding of bacteria is:
1. Histamine a potent vasoactive mediator, which can increase local blood flow and vascular permeability facilitating the arrival of inflammatory cells and serum antibodies to sites of bacterial infection.
TNF- and IL-6 are secreted by BMMCs after contact with opsonized Gram-negative or Gram-positive bacteria. TNF- released by MCs during their interaction with bacteria was shown to be crucial for the protective properties of these cells in the host.
Subfragments of fibrinogen and fibronectin generated following cleavage by plasmin, and the products of complement activation, C3a and C5a. Products of complement activation are crucial for MC activation in vivo following bacterial challenge.
In view of the close proximity of MCs to the mucosae, mucosal epithelial cells (ECs) play an important role in modulating MC responses to bacteria by secreting SCF, which in turn, might activate proximal MCs to produce proinflammatory cytokines, leading to amplified neutrophil recruitment and bacterial clearance. Increase in the production of SCF in mouse intestines was reported following infection with S. typhimurium.
Innate immune responses of MCs during bacterial infections in vivo
Mutant mice lacking MCs or defective c-kit protein, the receptor for SCF.
are available (WBB6F1-W/Wv mouse) and have been used to evaluate in vivo the specific contribution of MCs to the host defense against a given pathogen.
MCs are essential for mounting efficient innate immune responses against bacterial infections.
1.MC-deficient W/Wv mice are less efficient in clearing lung enterobacterial infections than wild-type mice or MC-reconstituted W/Wv mice by reducing neutrophil, lower levels of TNF- in these mice.
2. In innate immunity repetitive injections of SCF to C57BL/6 mice increased their number of MCs and improved the survival of these mice after the induction of acute septic peritonitis by cecal ligation and puncture (CLP).
W/Wv mice reconstituted with TLR4-mutated BMMCs had significantly higher mortality, which was associated with impaired neutrophil recruitment and cytokine production at the site of bacterial infection, compared with W/Wv mice reconstituted with TLR4-intact BMMCs
The precise role of the phagocytic capability of MCs remains undetermined, but is probably of minor impact compared with the bacterial killing mediated by professional phagocytes, such as neutrophils recruited by MC-derived mediators.
Clearly, one limitation of the data described above is that they are based on rodent MCs. The lack of a human model of MC deficiency. Future experiments can be envisaged
in vitro models of isolated mature human tissue MCs, instead of primary cultures of progenitors or cell lines.
the determination of the level of MC-derived mediators, such as tryptase, in the bronchoalveolar fluid (BAF) of patients after acute bacterial bronchial infections, or the search for the presence of bacteria bound to or present in MCs from the BAF of the same patients.
MCs might have deleterious effects during bacterial infections
The intrinsic capacity of MCs to induce marked pathological effects by the excessive or inappropriate release of inflammatory mediators might be significant in many infectious situations. The hypersecretion of inflammatory mediators by MCs triggered with bacterial components is exemplified by:
Shiga toxin, which is produced by the major intestinal pathogen Shigella dysenteriae. It has been shown that this toxin stimulates intestinal cells to release excessive amounts of LTC4 . Interestingly, MCs might be involved, at least in part, in this phenomenon. This is suggested strongly by a study from Pulimood et al. which showed that MCs in patients with shigella-related diarrhea probably release inflammatory mediators derived from arachidonic acid metabolites, as indicated by the increased number of lipid bodies in their cytoplasm. It is suggested that this hypersecretion might contribute to the pathophysiological response associated with dysentery, which involves excessive fluid and electrolyte secretion, resulting in diarrhea As a consequence, the pathogen might be able to disseminate itself throughout the environment, thereby facilitating its uptake by other hosts.2
Trigger of inflammation in Helicobacter pylori infection. An increase in the number of MCs has been described in the mucosa of patients with gastritis owing to H. pylori infection .In addition, it appears that some products of H. pylori might be able to induce the degranulation of MCs implicating these elements in the inflammatory process accompanying H.-pylori-related gastritis.
MCs might be implicated also in the pathogenesis of intestinal inflammation by Clostridium-difficile-derived toxins. Indeed, the participation of MCs in intestinal-fluid secretion and neutrophil infiltration mediated by toxin A has been confirmed in in vivo experiments, in which intestinal-fluid secretion and neutrophil recruitment were diminished significantly in MC-deficient W/Wv mice receiving toxin A, compared with normal mice
Bacterial subversion of the phagocytic machinery of MCs
Although MCs are able to phagocytose and kill various bacteria in the presence of serum opsonins, certain bacteria can subvert this capacity to their advantage in opsonin-poor environments. Specific receptors on MCs and their complementary ligands on the bacterial-cell surface are thought to be involved in such interactions.
At present, the best-described model for mast-cell recognition of microbes in nonopsonic conditions is the binding of BMMCs to E. coli and K. pneumoniae This interaction is mediated by the coupling of CD48, a glycosylphosphatidyl inositol-linked, mannose-containing receptor on the MC surface, to FimH, a mannose-binding lectin present on the filamentous adhesion organelles of the bacteria, known as type-1 fimbriae .
In serum-deficient conditions, these type-1 fimbriated strains of E. coli gain access into MCs without the loss of bacterial viability by a route distinct from the classical endosome¯lysosome pathway. In mice, CD48 is associated closely with sphingolipid¯cholesterol-enriched microdomains of the cell and CD48, which is localized to plasmalemmal caveolae in MCs, appears to facilitate the formation of bacteria-encapsulating caveolar chambers that are involved in bacterial entry into MCs. Of note, the internalized bacteria are contained in morphologically distinct compartments that fail to sequester oxygen radicals or become acidified, allowing the internalized bacteria to remain viable inside the cell Moreover, in such conditions, bacteria might even exit the MCs subsequently without significant loss of viability by again co-opting the cholesterol-rich microdomains .
Thus, in serum-deficient environments, such as the microenvironment of the urinary-tract mucosa, MCs can act as an insidious intracellular refuge for type-1 fimbriated E. coli and might contribute to the recurrence of infections.
Fig. 2. Comparative scheme of the behavior of type-1 fimbriated Escherichia coli in rodent mast cells (MCs) in (a) opsonin-dependent and (b) opsonin-independent conditions. (a) In the opsonin-dependent condition, adhesive interaction is mediated by serum components, such as complement or antibodies, and leads to internalization through the classical endocytic¯phagocytic pathway, which results in bacterial killing. (b) In the opsonin-independent condition, CD48, a mannosylated glycoprotein present on the MC membrane, binds FimH, a mannose-binding lectin present on the filamentous adhesion organelles of type-1 fimbriated bacteria, and this leads to internalization without bacterial killing and even, subsequent bacterial exit.
לחץ על התמונה להגדלה
Box 1. Mast cells might have beneficial and detrimental actions for the host during bacterial infections
Beneficial actions
![]()
Phagocytosis and killing of microbes in opsonin-dependent conditions.
Appropriate recruitment of immune cells, such as professional phagocytes.
Presentation of bacterial antigens to immune cells.
Regulation of adaptive immune responses.
Detrimental actions1
Intracellular reservoir for pathogens in opsonin-independent conditions.
Excessive recruitment of inflammatory cells, leading to tissue injury.
Excessive release of mediators, inducing edema, vascular stasis, cytotoxicity and fibrosis.
1 Some of the harmful actions are only postulated, based on the behavior of mast cells in other inflammatory conditions.
- אבחנות: כלכלת בריאות
- קטגוריות: מאמרים
מידע נוסף לעיונך
כתבות בנושאים דומים

עליה של עשרות אחוזים בשיעורי התמותה על-רקע היריון בשנים האחרונות בארצות הברית (JAMA Netw Open)
בארצות הברית דווח על שיעורי התמותה על-רקע היריון הגבוהים ביותר מבין המדינות בעלות הכנסה-גבוהה, עם למעלה מ-6,000 מקרי תמותה מדווחים בין 2018 עד 2022, כך על-פי נתונים שפורסמו בכתב העת JAMA Network Open. מניתוח הנתונים עלה כי בילידות אלסקה ובאמריקאיות ממוצא אינדיאני תועדו שיעורי התמותה הגבוהים ביותר. מחקר החתך התבסס על נתונים ארציים של המרכז […]

אימוני אינטרוול בעצימות גבוהה מפחיתים סיכון להידרדרות תפקוד כלייתי בקשישים (J Am Society Nephrol)
אימוני אינטרוול בעצימות גבוהה (High-Intensity Interval Training, או HIIT) תחת השגחה לאורך חמש שנים עשויים להפחית את הסיכון להידרדרות מהירה בקצב הפינוי הגלומרולארי המשוער בקשישים בגילאי 70-77 שנים, כך עולה מנתונים חדשים שפורסמו בכתב העת Journal of the American Society of Nephrology. ברקע למחקר מסבירים החוקרים כי למרות היתרונות הבריאותיים הרבים של פעילות גופנית, אין […]

האם המהפכה הטיפולית בהשמנת יתר תפרוץ את חומת הכלכלה? פרופ' רז ופרופ' שטרן
לפניכם הרצאה ודיון שהתקיים בין פרופ’ איתמר רז ופרופ’ נפתלי שטרן בנושא השפעות החידושים הטיפוליים בתחום ההשמנה. https://vimeo.com/820235359

בעקבות המלצות ועדת הסל - ראיון מיוחד עם פרופ' נדב דווידוביץ
על רקע פרסום המלצות ועדת הסל ביקשנו לבדוק עם פרופ’ נדב דווידוביץ, חבר בוועדת הסל, כמה סוגיות שקשורות למתודולוגיה ולאופן קבלת ההחלטות בוועדה.

מאזן עלות-תועלת של הטיפול ב-Dapagliflozin במחלת כליות כרונית (Clin J Am Soc Nephrol)
אנליזה חדשה של מחקר הCKD-DAPA מראה שהטיפול ב-dapagliflozin (פורסיגה) במחלת כליות כרונית הינו משתלם מבחינת מאזן עלות-תועלת בבריטניה, גרמניה וספרד. ברקע למחקר מסבירים החוקרים כי מחלת כליות כרונית (Chronic Kidney Disease CKD) מהווה נטל משמעותי על המטופלים ועל שירותי הבריאות, בעיקר בהגעה לאי ספיקת כליות סופנית כשהמטופלים נדרשים לטיפול כלייתי חליפי. מחקר ה-DAPA-CKD הראה שהטיפול ב-dapagliflozin, […]

איך זה עבר בשקט ומתחת לרדאר? עלות ההשמנה בישראל - 20 מיליארד ש''ח לשנה (הודעת החברה להשמנה)
כבר לפני יותר משנה הושלם דו”ח מקיף, המנתח את השלכות מחלת ההשמנה על חוסן מדינת ישראל, עם השוואות לעולם ותכנית ברורה למדיניות בריאות מסודרת ולרגולציה מותאמת. נחשף היופ, בתום הכנס השנתי של החברה הישראלית לחקר וטיפול בהשמנת יתר של הר”י ד”ר דרור דיקר (בתמונה) : “נהוג להתחמק מדיון אמיתי במחלת ההשמנה, בשל סטיגמה […]

ארגוני הרוקחים הגישו בג''צ בשל סירוב משרד הבריאות להכליל שירותי ייעוץ רוקחי יזום בסל הבריאות
קואליציית ארגוני הרוקחות הגישה הבוקר עתירה לבג”ץ בנושא סירוב משרד הבריאות להכליל שירותי ייעוץ רוקחי יזום בסל הבריאות, בטענה שהייעוץ הינו כבר חלק מהסל. ארגון הרוקחות הגיש את הייעוץ התרופתי היזום כטכנולוגיה להכללה בסל שירותי הבריאות הממלכתי לשנת 2023. מטרת השירות הינה לאפשר לרוקחים במפגש ייעודי עם מטופלים, שלא אגב ניפוק תרופה מסוימת, לבצע הדרכת […]

ב-2021 היקף התמיכות בקופות החולים הסתכם בכ-7 מיליארד ש''ח, מהם 2.7 בגין הוצאות הקורונה (דו''ח משרד הבריאות)
משרד הבריאות מפרסם דוח מסכם על פעילות קופות החולים לשנת 2021 היקף התמיכות בקופות החולים הסתכם לסך של כ-7 מיליארד ש”ח, המהווים כ-12% מעלות סל הבריאות. מתוך סכום זה כ-2.7 מיליארד ש”ח הם תמיכות בגין הוצאות הקורונה. השתתפויות עצמיות ממבוטחים בסל בגין שירותים עלו בשיעור של 6.1% כללית ומכבי מציגות הון עצמי חיובי, לעומת מאוחדת […]

הורביץ בישיבה הראשונה של ועדת הסל: לראשונה תקציב הרחבת הסל מקובע במסגרת תקציב המדינה
דיון פתיחה של הוועדה הציבורית להרחבת סל שירותי הבריאות לשנת 2023 היום (ראשון ה-18.9.22) התכנסה הוועדה הציבורית להרחבת סל שירותי הבריאות לשנת 2023 לישיבתה הראשונה במשרד הבריאות במרכז הרפואי שיבא תל השומר בהשתתפות שר הבריאות, ניצן הורוביץ ומנכ”ל משרד הבריאות פרופ’ נחמן אש. שר הבריאות, ניצן הורוביץ: “בבסיס עבודת הוועדה, ובעצם מהות עבודתה, היא מימוש […]












השאירו תגובה
רוצה להצטרף לדיון?תרגישו חופשי לתרום!